Introductions Although intratumor heterogeneity and clonal evolution have been inferred in multiple myeloma (MM), this was largely focused at the bulk tumor population level. Single-cell analysis is of significant importance in delineating the exact phylogeny of subclonal population and in discovering subtle diversification. Here, we identified the clonal architecture of different time points using multi-gene fluorescence in situ hybridization (mFISH) at single cell level, and explored the prognostic values of different clonal evolution patterns in MM.
Methods We performed mFISH in 129 longitudinal samples of 57 MM patients. All the patients had newly-diagnosed and relapsed paired samples, and 12 patients had cytogenetic evaluation for more than two time points. An expanded cohort of 188 MM patients underwent conventional FISH (cFISH) to validate the cytogenetic evolution in bulk tumor level.
Results 43 of 57 patients (75.4%) harbored three or four cytogenetic clones at diagnosis. We delineated the phylogeny of subclonal tumor population in each patient and established robust trends for the timing of temporal acquisition in the whole cohort using the pairwise precedence. 13q deletion and the first 1q gain tended to be earlier cytogenetic alternation, whereas 16q and 17p deletion were acquired later. The sequence of 13q deletion and 1q21 gain occurrence was identified in 23 patients by the single-cell analysis. 1q21 gain and 13q deletion each occurred first in 12 and 11 patients respectively. Strikingly, patients in whom 13q deletion was acquired first showed a significantly worse survival than 1q21 gain-first patients (median OS 32.9 vs. 71.2 months, p=0.010).
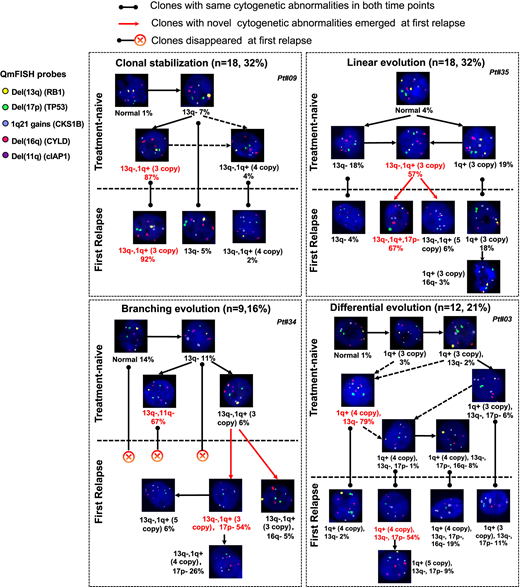
We inferred the most likely ancestral relationships between subclones and derived the evolutionary architecture in each patient. Four distinct evolutionary patterns were identified (Figure 1). 18 of 57 (31.6%) patients showed clonal stabilization. These patients were characterized by no novel subclones emerging and no existed subclones disappearing at relapse. Differential evolution was observed in 12 patients, where clonal dynamics resulted from a change in predominant clone from presentation to relapse. The major clone at diagnosis disappeared or decreased to a minor clone while a subclone showed growth advantage and turned to be a major clone at relapse. We found evidence of branching evolution in 9 patients. Here, one or more clones harboring novel cytogenetic abnormalities emerged between the early and late time points, whereas some disappeared. The remainder of patients demonstrated a linear evolution pattern (18/57, 31.6%). The predominant clones acquired one or more novel cytogenetic abnormalities at the later time point. Patients with clonal stabilization had a significantly improved OS than those with other evolutionary patterns (median OS, 71.2 vs. 39.7 vs. 35.2 vs. 25.5 months, for stable, differential, branching and linear patterns, respectively, p=0.001). However, there is no difference in sampling interval among four evolutionary patterns (p=0.131). Therefore, the survival differences were mostly attributable to a significantly shorter failure free survival from relapse (p<0.001).
In order to evaluate the accuracy of abnormalities detection by mFISH, we performed cFISH in these 57 MM patients. Cell fractions of cytogenetic abnormalities detected by mFISH were significantly correlated with that detected by cFISH (p<0.001). Besides, a high degree of consistency and complementarity across cFISH and mFISH was observed in evaluation of cytogenetic evolution pattern in MM. Then we expanded our cohort to 188 patients to further discuss the prognostic value of cytogenetic evolution. Survival from relapse were greater influenced by the presence of high-risk aberrations at relapse (HR=2.07) rather than present at diagnosis (HR=1.55). There was no difference in OS for patients who had primary high-risk aberrations at diagnosis compared with those who developed high-risk aberrations after relapse (p=0.800).
Conclusions These findings suggest that mFISH is a valuable tool for the analysis of clonal phylogeny and evolution pattern of critical cytogenetic aberrations. Patients may benefit from the repeated cytogenetic evaluation, especially for the risk stratification of survival after relapse. Personalized treatment strategy is required for MM patients based on their clonal evolution patterns.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.